Standard Formation Reaction of Solid Aluminum Hydroxide
Double replacement Please tell about this free chemistry software to your friends. Write a balanced chemical equation for the standard formation reaction of solid aluminum hydroxide.
Solved Write A Balanced Chemical Equation For The Standard Chegg Com
The net balance equation for the reaction is.

. Write a balanced chemical equation for the standard formation reaction of solid sodium hydrogen. Beta alumina may also be synthesised directly from the various aluminium hydroxide AlOHJ. AlOH3s 3 Haq -- Al3aq 3 H2OI Wiki User.
Write a balanced chemical equation for the standard formation reaction of solid aluminum hydroxide Al OH. The chemical behavior of aluminum in dilute solutions presents some serious experimental difficulties. Helpful 0Not Helpful 0 Add a Comment.
Check the balance The thermal decomposition of aluminium hydroxide to produce aluminum oxide and water. View the full answer. First O atom number on both sides of the equation.
Therefore the unbalanced standard formation reaction is. Most reactions involving aluminum and hydroxide proceed slowly. D- х.
C p heat capacity JmolK H standard enthalpy kJmol S standard entropy JmolK t temperature K 1000. This reaction can also be categorized as double displacement reaction. Direct link to this balanced equation.
H H 29815 At Bt 2 2 Ct 3 3 Dt 4 4 Et F H. 3H 2 SO 4 2Al OH ------ Al 2 SO 4 3 6H 2 O. A solid vanadium V oxide V 2 O 5 b solid aluminum hydroxide AlOH 3 Heat of formation Heat of formation.
Two of these Which of the compounds below is an example of a network solid. Who are the experts. Experts are tested by Chegg as specialists in their subject area.
The reaction formation of the. We review their content and use your feedback to keep the quality high. We find 2 atoms on the right side and 1 in the left side so we put 2HgO instead of HgO to make the number of O equals on each side.
See answer 1 Best Answer. Standard state of sulfur is S 8 s Example Write the balanced chemical equation for the standard formation of. The balanced chemical equation for the standard formation reaction of solid glucose is.
To maximise the amount of 6 -alumina formed a second heat treatment step is required. The formation of solid glucose occurs by the reaction of carbon hydrogen and oxygen. AluminumAcetate 3 3 AmmoniumHydroxide AluminumHydroxide 3 3 AmmoniumAcetate Reaction type.
So the equation become Hg. The objective is to precipitate aluminum hydroxide per the following reaction which I believe to be correct. Hg l O2 g HgO s and we start to compare.
The change in enthalpy when 1 mole of the compound forms from its constituent elements in their standard states for methane CH 4 This value relates to the. Cl_2g Als - AlCl_3s Balancing it to ensure that we form one mol of product as it is defined for a standard formation reaction we write. Al2SO43 HCO3- 2AlOH3 SO4-- I do not want Al2SO43 H2O 2AlOH3 H2SO4.
One mole of aluminum sulfate and six moles of water are produced when three moles of sulfuric acid are combined with two moles of aluminum hydroxide. From the chemical formula of solid aluminium hydroxide we can say that it is formed by the reaction question_answer Q. Write a balanced chemical equation for the standard formation reaction of solid aluminum hydroxide AlOH3 Steel is considered to be an A.
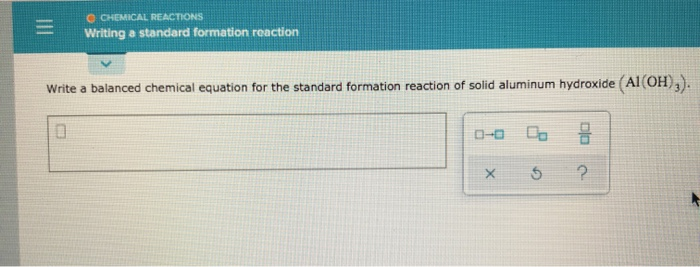
Glucose is a simple sugar of a class of carbohydrates known as monosaccharidesIt is the principal metabolite for energy production in the human body. Formed through the high temperature solid state reaction of a-alumina with soda and lithia which results in a mixture of 6-and 6-alumina phases. CHEMICAL REACTIONS Writing a standard formation reaction Write a balanced chemical equation for the standard formation reaction of solid aluminum hydroxide Al OH.
2Al OH 3 Al 2 O 3 3H 2 O. S Aln t Bt Ct 2 2 Dt 3 3 E 2t 2 G. The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements with all substances in their standard statesThe standard pressure value p 10 5 Pa 100 kPa 1 bar is recommended by IUPAC although prior to 1982 the value 100 atm 101325 kPa was.
Aluminum naturally exists at 25 C and 1 atm as a solid. Colorbluebarulstackrel 32 Cl_2g Als - AlCl_3s And this has an. Aluminum hydroxide AlH3O3 CID 10176082 - structure chemical names physical and chemical properties classification patents literature biological activities.
Standard free-energy values for the common solid forms of aluminum hydroxide also disagree. Check Save For Lates 2020 M A MacBook BO 44 14 3 7 2 4 6 8 0. 100 19 ratings Aluminium Hydroxide can be formed from Aluminium and Oxygen and Hydr.
Chemistry questions and answers. This reaction takes place at a temperature of over 575C.
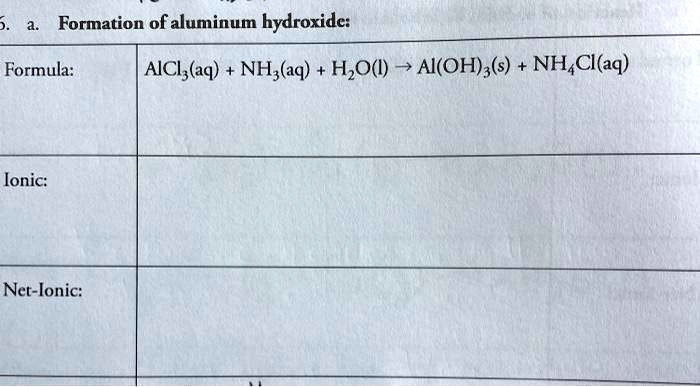
Solved Formation Of Aluminum Hydroxide Formula Aici Aq Nh Aq Hzo Ai Oh S Nh Cl Aq Ionic Net Ionic
Solved Write A Balanced Chemical Equation For The Standard Chegg Com

Comments
Post a Comment